尊敬的用戶您好,這是來自FT中文網的溫馨提示:如您對更多FT中文網的內容感興趣,請在蘋果應用商店或谷歌應用市場搜尋「FT中文網」,下載FT中文網的官方應用。
“Pig hearts to be tested in humans” read a headline in the Financial Times in September 1995. The article quoted confident predictions that porcine hearts, genetically engineered to avoid rejection, would be transplanted into patients the following year — helping to ease the worldwide shortage of human organs.
1995年9月,FT發表了一篇題爲「豬心臟將在人體中進行測試」的文章。這篇文章引用了有信心的預測,透過基因工程來避免排斥反應的豬心臟將在次年被移植到病人體內,這有助於緩解全球人體器官短缺的問題。
More than 25 years passed before that forecast finally came true. Last month surgeons at the University of Maryland School of Medicine in Baltimore successfully replaced the failing heart of a 57-year-old man with a fresh one from a pig with 10 gene edits. Four weeks later the patient, David Bennett, continues to recover with no signs of rejection.
25年後,這一預測終於成真。上個月,位於巴爾的摩的馬里蘭大學醫學院的外科醫生們成功地用一頭帶有10個基因編輯的豬的新鮮心臟替換了一名57歲男子衰竭的心臟。四個星期後,病人大衛·班奈特(David Bennett)繼續康復,沒有出現排斥反應的跡象。
Xenotransplantation — replacing human organs with those from other species, typically referred to as “xeno” by scientists — has followed cycles of hope and disappointment dating back at least to 1984 when surgeons in California gave Baby Fae, born with a lethal cardiac defect, a baboon’s heart which failed after three weeks.
異種移植——用科學家通常稱之爲「異種」的其他物種的器官取代人類器官——經歷了希望和失望的循環,至少可以追溯到1984年,當時加州的外科醫生給有致命心臟缺陷的嬰兒菲(Fae)移植了一隻狒狒的心臟,這顆心臟在三週後衰竭。
Now, after decades of anticipation, a new era of medical innovation could be within grasp, propelled by advances in gene editing, including Crispr technology, animal cloning and immunology. At the same time fears that transplants could transfer viruses from animals to humans, which almost killed xeno research in the late 1990s, have eased.
如今,經過幾十年的期待,在Crispr技術、動物克隆和免疫學等基因編輯技術的進步推動下,醫療創新的新時代可能即將到來。與此同時,對移植會將病毒從動物傳染給人類的擔憂也有所緩解。在20世紀90年代末,這種擔憂幾乎扼殺了異種動物研究。
The Maryland cardiac team surprised the world of medicine with its announcement of a transplant of a pig’s organ into a living patient, because this did not mark the start of a formal clinical trial agreed with the US Food and Drug Administration and flagged in advance. Instead the FDA gave a one-off “emergency use authorisation”.
馬里蘭州的心臟研究小組宣佈將豬的器官移植到活著的病人身上,這一訊息震驚了醫學界,因爲這並不標誌着與美國食品和藥物管理局(US Food and Drug Administration,簡稱fda)達成協議並提前通知的正式臨牀試驗的開始。相反,FDA給予了一次性的「緊急使用授權」。
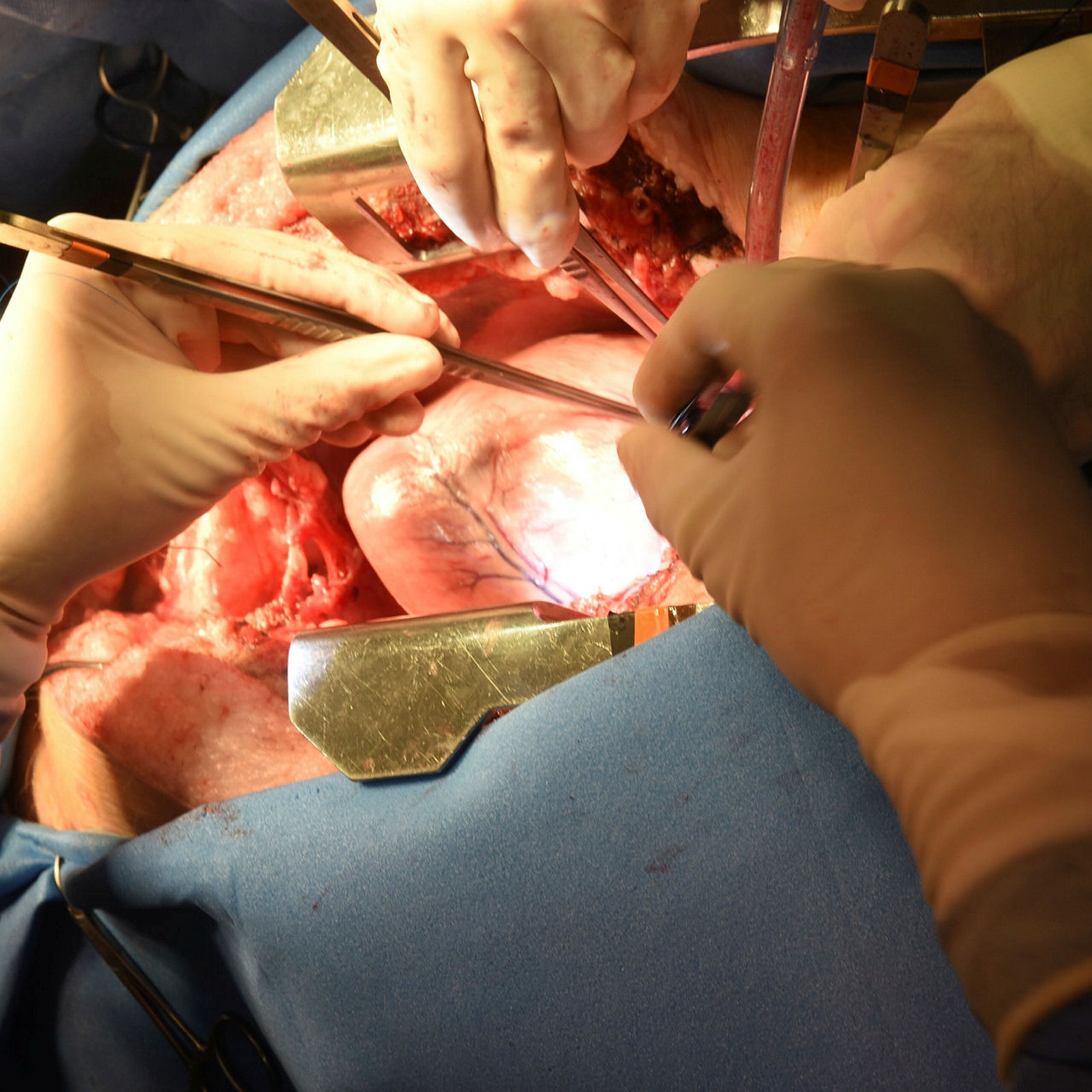
A surgeon operates on a pig prior to the removal of its heart
University of Maryland School of Medicine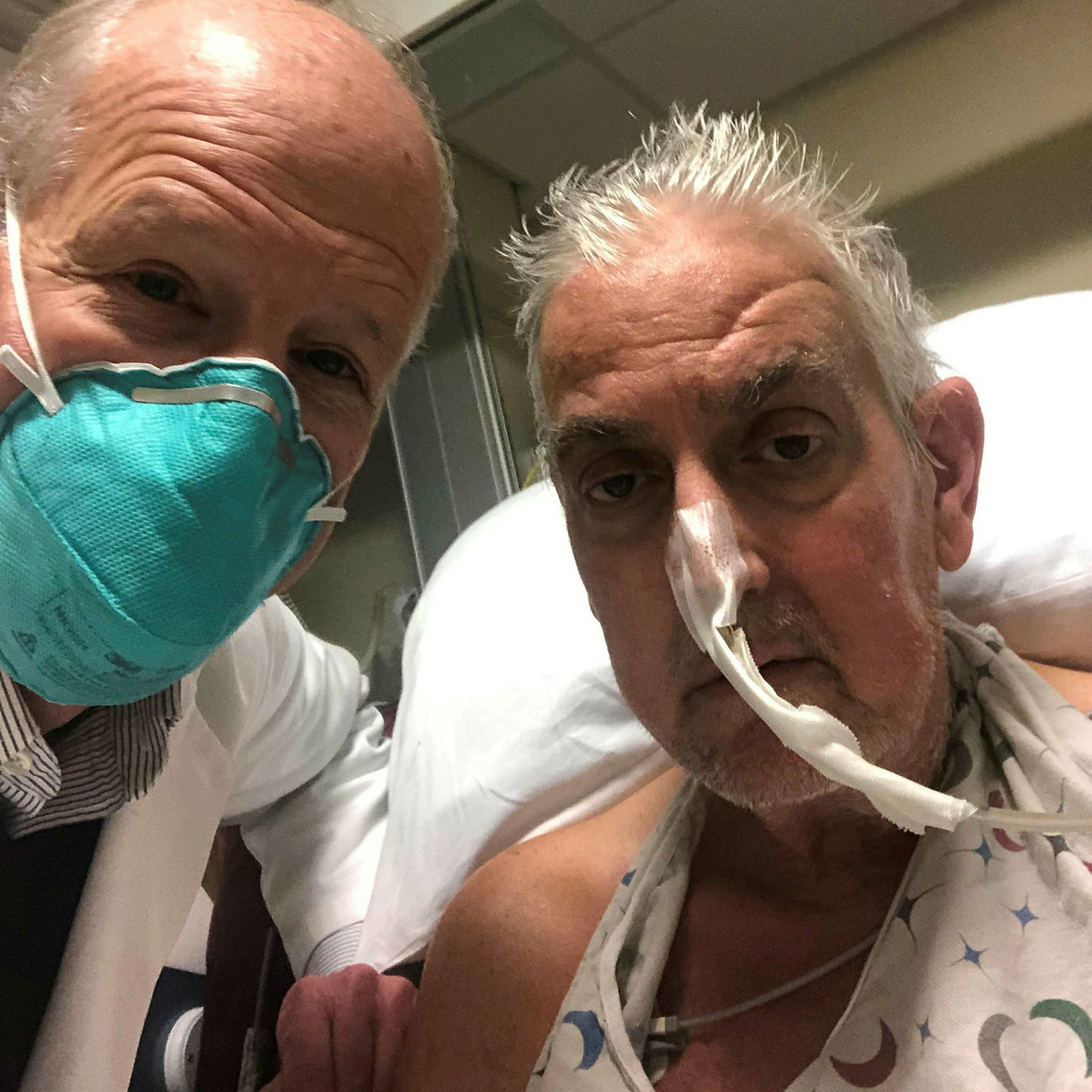
Bartley Griffith, left, was the lead surgeon for patient David Bennett, who received a pig heart at the University of Maryland Medical Center last month
University Of Maryland School Of Medicine

“The FDA had never before given permission for anything like this,” says Muhammad Mohiuddin, professor of surgery and director of Maryland’s xeno programme. “The [authorisation] was granted because it was the only option for saving our patient, who was hospitalised with a life-threatening medical condition and was not eligible for a normal heart transplant.” The Maryland team received permission on New Year’s Eve and carried out the operation a week later.
「FDA以前從未批准過這樣的事情,」外科教授、馬里蘭州異種項目主任穆罕默德·穆希丁(Muhammad Mohiuddin)說。「授予(許可)是因爲這是拯救我們的病人的唯一選擇,他當時因危及生命的疾病住院,沒有資格進行正常的心臟移植。」馬里蘭州的研究小組在新年前夜獲得了許可,並在一週後進行了手術。
Mohiuddin, who has been involved in xenotransplant research since coming to the US from Pakistan 30 years ago, says Bennett’s progress after the operation will inevitably be slow. “He was in very bad shape, having been bedridden for three months. We’ve done the equivalent of putting a new Ferrari engine in the 1960s Ford. The engine is working fine but the body will take time to adjust.
穆希丁30年前從巴基斯坦來到美國,一直從事異種移植研究。他說,班尼特手術後的進展將不可避免地緩慢。「他在牀上躺了三個月,情況非常糟糕。我們所做的,相當於在20世紀60年代的福特汽車上安裝了一個新的法拉利引擎。引擎運轉良好,但身體需要時間來調整。」
“The biggest thing we recognise in him is his will to live,” he continues. “Physicians and scientists can’t do anything for a patient without that determination.”
「我們在他身上認識到的最重要的是他活下去的意志,」他繼續說。「沒有這種決心,醫生和科學家無法爲病人做任何事。」
Xeno researchers and doctors have been carrying out pre-clinical research for several years, transplanting pig hearts and kidneys into dozens of non-human primates — mainly baboons. Now they are impatient to move on to people.
異種的研究人員和醫生已經進行了幾年的臨牀前研究,將豬的心臟和腎臟移植到數十種非人類靈長類動物——主要是狒狒身上。現在,他們迫不及待地要把注意力轉移到人身上。
“We’re becoming better and better primate doctors, but of course that is not the goal,” says Robert Montgomery, head of the NYU Langone Transplant Institute, who himself received a new heart in 2018 from someone who had died of a heroin overdose. “I think we’re as ready as we’re going to be to move into [human trials]. It’s still a big jump, but we’re gaining confidence.”
紐約大學朗格尼移植研究所(NYU Langone Transplant Institute)所長羅伯特·蒙哥馬利(Robert Montgomery)說:「我們正在成爲越來越優秀的靈長類醫生,但這當然不是我們的目標。」他自己在2018年接受了一位死於海洛因過量的人的新心臟。「我認爲我們已經準備好進入(人體試驗)。這仍然是一個很大的飛躍,但我們正在增強信心。」
Bennett’s “proof of concept” surgery could speed up the regulatory approval process. But there is also pressure from demographics. As populations grow older, the shortage of organs will probably grow more acute. In the US the gap between those waiting for organs and the number of transplants completed is widening. In 2002, just over 42,000 people needed transplant organs while today there are 66,216 on the waiting list.
班奈特的「概念證明」手術可以加快監管部門的審批過程。但也有來自人口結構的壓力。隨著人口年齡的成長,器官的短缺可能會越來越嚴重。在美國,等待器官的人和完成移植的數量之間的差距正在擴大。2002年,只有42,000多人需要移植器官,而今天有66,216人在等待。
Organs from animals that have been specifically bred for transplant purposes also have advantages in consistency and quality over those from humans, according to David Ayares, chief scientist at Revivicor, the biotech company that supplied Bennett’s new heart.
提供班奈特新心臟的生物技術公司Revivicor的首席科學家大衛·阿亞里斯(David Ayares)表示,與來自人類的器官相比,專門爲移植目的培育的動物的器官在一致性和質量上也有優勢。
“Xenotransplants will be the solution to the organ shortage crisis,” he says. “We’ll be able to provide viable organs from young, fresh animals which can be grown to the size suited to the recipient.”
「異種移植將是解決器官短缺危機的辦法,」他說。「我們將能夠從年輕、新鮮的動物身上提供可存活的器官,這些器官可以長到適合接受者的大小。」
Scaling up to humans
擴大到人類
While they wait for regulators to approve clinical trials with real patients, researchers are beginning to work with “pre-clinical human models,” or “decedents” — people who are brain-dead but whose bodily functions can be maintained for a few days through life support machines.
在等待監管機構批准用真正的病人進行臨牀試驗的同時,研究人員已經開始使用「臨牀前人體模型」,也就是「死者」——那些腦死亡的人,他們的身體功能可以透過生命維持機維持幾天。
In three procedures last autumn, one at the University of Alabama at Birmingham (UAB) and two at New York University Langone Health, surgeons transplanted pigs’ kidneys into decedents.
Although these experiments were designed only to last for a few days before the decedents’ life support systems had to be switched off, the kidneys worked well without acute rejection, processing blood and producing urine.
雖然這些實驗被設計爲只能持續幾天,然後死者的生命維持系統就必須關閉,但腎臟工作良好,處理血液和產生尿液,沒有急性排斥反應。
The UAB team, led by Jayme Locke, operated on 57-year-old Jim Parsons who was fatally injured in a motorbike accident. He was a registered donor but his organs were not suitable for transplantation, so his family agreed to donate his body for research.
由傑米·洛克(Jayme Locke)領導的UAB團隊爲57歲的吉姆·帕森斯(Jim Parsons)做了手術,他在一次摩托車事故中受了致命的傷。他是一名註冊捐贈者,但他的器官不適合移植,所以他的家人同意將他的遺體捐獻給研究。
“Trying to ascertain [donated organ] function in the face of brain death is always going to be challenging,” Locke says. “Ultimately we will need to move into a phase 1 clinical trial in which we transplant these kidneys into a living human being where the environment is more favourable for kidney recovery.”
洛克說:「面對腦死亡,試圖確定[捐贈的器官]的功能總是具有挑戰性的。最終,我們需要進入第一階段的臨牀試驗,我們將這些腎臟移植到一個活的人身上,那裏的環境更有利於腎臟的恢復。」


The FDA is insisting that the xeno teams build up more pre-clinical data by continuing to transplant into baboons before they start clinical trials. Locke hopes for approval by the end of this year of a phase 1 study. This might involve 10 to 20 people for whom there are no human donors, followed by an extensive phase 2 trial with a few hundred participants.
FDA堅持異種動物研究小組在開始臨牀試驗前透過繼續移植到狒狒身上來建立更多的臨牀前數據。洛克希望在今年年底前獲得第一階段研究的批准。這可能涉及到10到20個沒有人類捐贈者的人,然後進行大規模的2期試驗,有幾百名參與者。
Although some of the earliest xeno work in the 20th century was done with primate donors, particularly baboons, by the 1990s researchers had settled on pigs as the best animals to use. Despite the evolutionary distance between pigs and humans — our last common ancestor lived about 80mn years ago when mammals still lived in the shadow of dinosaurs — the two species are remarkably similar in size and physiology.
儘管20世紀最早的一些異種動物研究是用靈長類動物,尤其是狒狒做的,但到了20世紀90年代,研究人員已經確定豬是最適合使用的動物。儘管豬和人類在演化上存在差異——我們最後的共同祖先生活在8000萬年前,當時哺乳動物還生活在恐龍的陰影下——但這兩個物種在體型和生理上非常相似。
A big safety issue 25 years ago was the fear of inadvertently transferring potentially fatal viruses — and particularly a group called porcine endogenous retroviruses (Pervs) — from pigs to people with the transplanted organs. Subsequent experiments have suggested that this concern was overblown and that animals reared in pathogen-free facilities do not pose a risk of infection to human recipients.
25年前的一個重大安全問題是,人們擔心可能致命的病毒——尤其是一組被稱爲豬內源性逆轉錄病毒(Pervs)的病毒——會在不經意間從豬身上轉移到被移植器官的人身上。隨後的實驗表明,這種擔憂被誇大了,在無病原體設施中飼養的動物不會對人類接受者構成感染的風險。
“Our animals are bred and reared behind a sterile barrier,” says Locke. “They undergo quarterly testing for zoonotic infections and specifically Pervs . . . The level of scrutiny for a pathogen-free pig facility in many ways far exceeds what is required of a human-to-human transplant.”
「我們的動物是在無菌屏障後面繁殖和飼養的,」洛克說。「它們每季度都要接受人畜共患傳染病的檢測,特別是Pervs......。 對無病原體豬設施的審查水準在許多方面遠遠超過了對人與人之間移植的要求。」
UK scientists played an important role in early xenotransplant research but by the early 2000s the main action had moved to the US. The leading biotech company in the field is Revivicor, based in Blacksburg, Virginia, which developed the animals behind the latest xenotransplants into humans. Revivicor was spun out of PPL Therapeutics, the Scottish animal cloning company behind Dolly the Sheep, in 2003, and was bought in 2011 by United Therapeutics.
英國科學家在早期異種移植研究中發揮了重要作用,但到21世紀初,主要行動轉移到了美國。這一領域的領先生物技術公司是維吉尼亞州布萊克斯堡的Revivicor公司,該公司開發了最新的異種移植到人類體內的動物。2003年,Revivicor從蘇格蘭動物克隆公司PPL Therapeutics剝離出來,並於2011年被聯合治療公司(United Therapeutics)收購。
The University of Maryland and UAB teams used Revivicor pigs with 10 genetic modifications, though the company and its academic collaborators refuse to disclose how the gene editing was carried out for competitive reasons.
馬里蘭大學(University of Maryland)和UAB團隊使用了帶有10個基因修改的Revivicor豬,但該公司及其學術合作伙伴出於競爭原因拒絕透露基因編輯是如何進行的。
Four pig genes were disabled. Three of them prevent the animals adding carbohydrate molecules to cells in their organs which might cause the recipient’s body to reject the transplant. The fourth “knockout” is a pig growth hormone gene, to prevent the organ growing too large in the recipient.
豬的四個基因被關閉。其中三個防止動物向器官細胞中新增碳水化合物分子,這可能會導致受者的身體排斥移植。第四個被移除的是一個豬生長激素基因,以防止器官在受體中長得太大。
The other six changes are “knock-ins” — human genes added to the pigs to prevent unwanted blood clotting, excessive inflammation and attack by antibodies. Their combined effect is to cut the risk that the human immune system will reject the porcine organs, both immediately and in the longer term.
另外六個變化是「引入基因」——人類基因被新增到豬身上,以防止不必要的血液凝結、過度炎症和抗體攻擊。它們的聯合作用是降低人類免疫系統對豬器官的排斥風險,無論是直接的還是長期的。
Revivicor’s Ayares expects that pigs with the same 10 gene edits will be suitable donors for several different organs, though not all. “They are fine for heart and kidneys,” he says, “but liver and lungs have proven more challenging. For lungs we’re likely to have to tweak the genetic modifications.”
Revivicor公司的阿亞里斯預計,擁有同樣10個基因編輯的豬將是幾種不同器官的合適捐贈者,但不是全部。「它們對心臟和腎臟很好,」他說,「但事實證明,對肝臟和肺更有挑戰性。對於肺部,我們可能必須調整基因修改。」
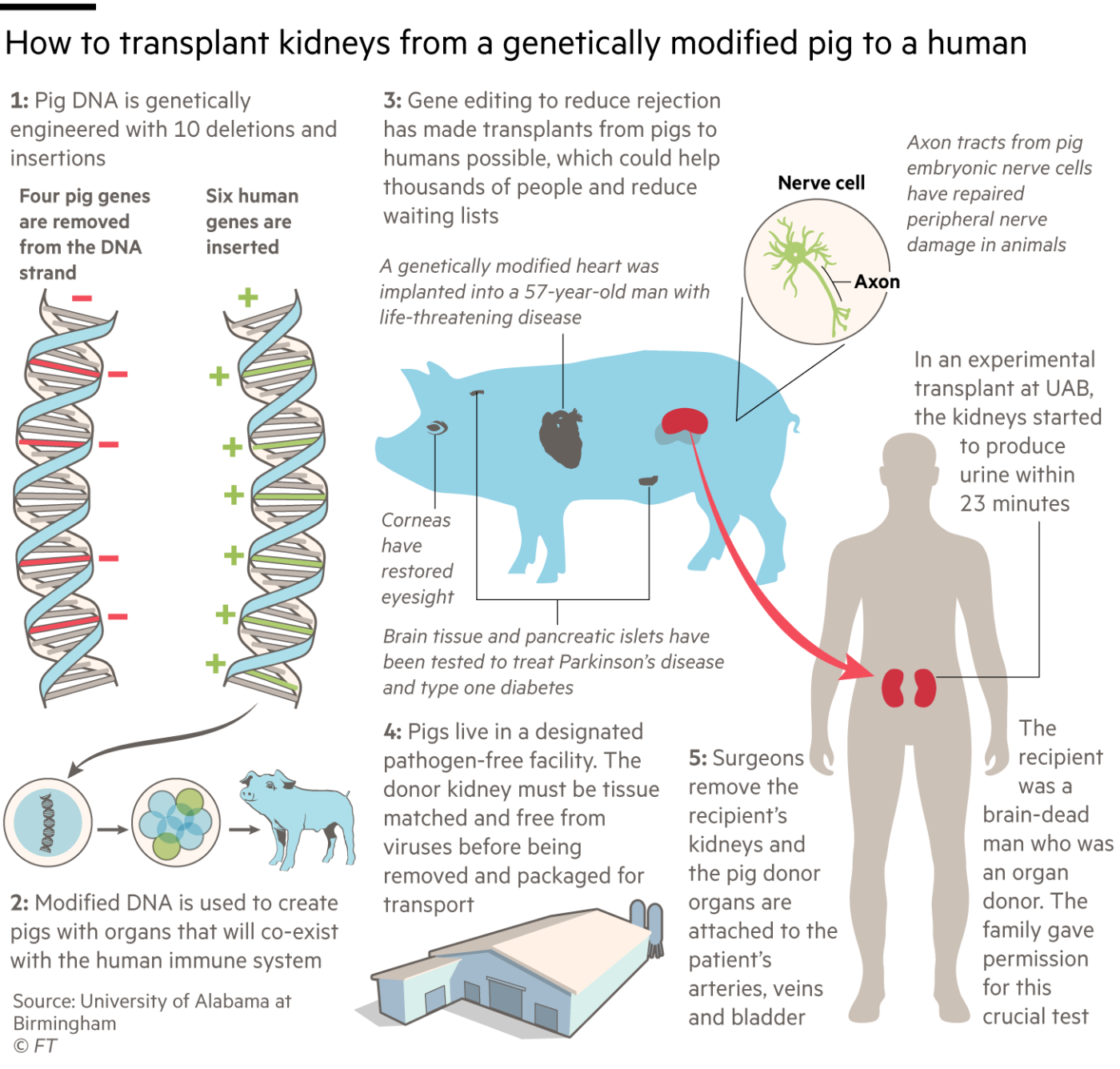
Montgomery’s NYU team is using an earlier Revivicor pig with just a single genetic modification, removing a molecule called alpha-gal, which is believed to be the most important cause of immune rejection in xenotransplants. His procedure also involves fusing the pig’s kidney with its thymus gland, which helps to control the immune system, before transplantation.
蒙哥馬利的紐約大學研究小組使用的是早期的Revivicor豬,只進行了一次基因修改,去除了一種叫做alpha-gal的分子,這種分子被認爲是異種移植免疫排斥的最重要原因。他的手術還包括在移植前將豬的腎與胸腺融合,這有助於控制免疫系統。
A newer xeno company is eGenesis, founded by Harvard University scientists in 2015, which collaborates with medical teams at Massachusetts General Hospital, Duke University and the University of Wisconsin. Its pigs have many of the same modifications as Revivicor’s, though one difference is that eGenesis also uses the latest Crispr gene editing technology to delete all retrovirus genes incorporated in the animal’s genome, eliminating the risk of Pervs infecting people.
一個較新的異種公司是eGenesis,由哈佛大學(Harvard University)的科學家在2015年成立,它與馬薩諸塞州總醫院(Massachusetts General Hospital)、杜克大學(Duke University)和威斯康星大學(University of Wisconsin)的醫療團隊合作。該公司的豬與Revivicor的豬有許多相同的基因修改,但一個不同之處在於,eGenesis還使用最新的Crispr基因編輯技術,刪除動物基因組中包含的所有逆轉錄病毒基因,消除了Pervs感染人類的風險。
“Our focus now is on getting long-term survival in non-human primate models,” says Mike Curtis, eGenesis head of R&D. “We have two [animal] recipients over 400 days post-transplant in our kidney programme . . . We are poised to be in the clinic within the next couple of years.”
「我們現在的重點是在非人類靈長類動物模型中獲得長期生存,」eGenesis研發主管邁克•柯蒂斯(Mike Curtis)說。「在我們的腎臟移植項目中,有兩名(動物)受者在移植後存活超過400天……我們準備在未來幾年內進行臨牀試驗。」
If the clinical trials go well, the companies foresee no problem in increasing production to supply tens or even hundreds of thousands of animals a year for xenotransplantation. “The agriculture industry has shown us how to scale up pig production,” says Curtis.
如果臨牀試驗進展順利,這兩家公司預計增加產量,每年爲異種移植提供數萬甚至數十萬只動物是沒有問題的。柯蒂斯說:「農業產業已經向我們展示瞭如何擴大豬的生產規模。」
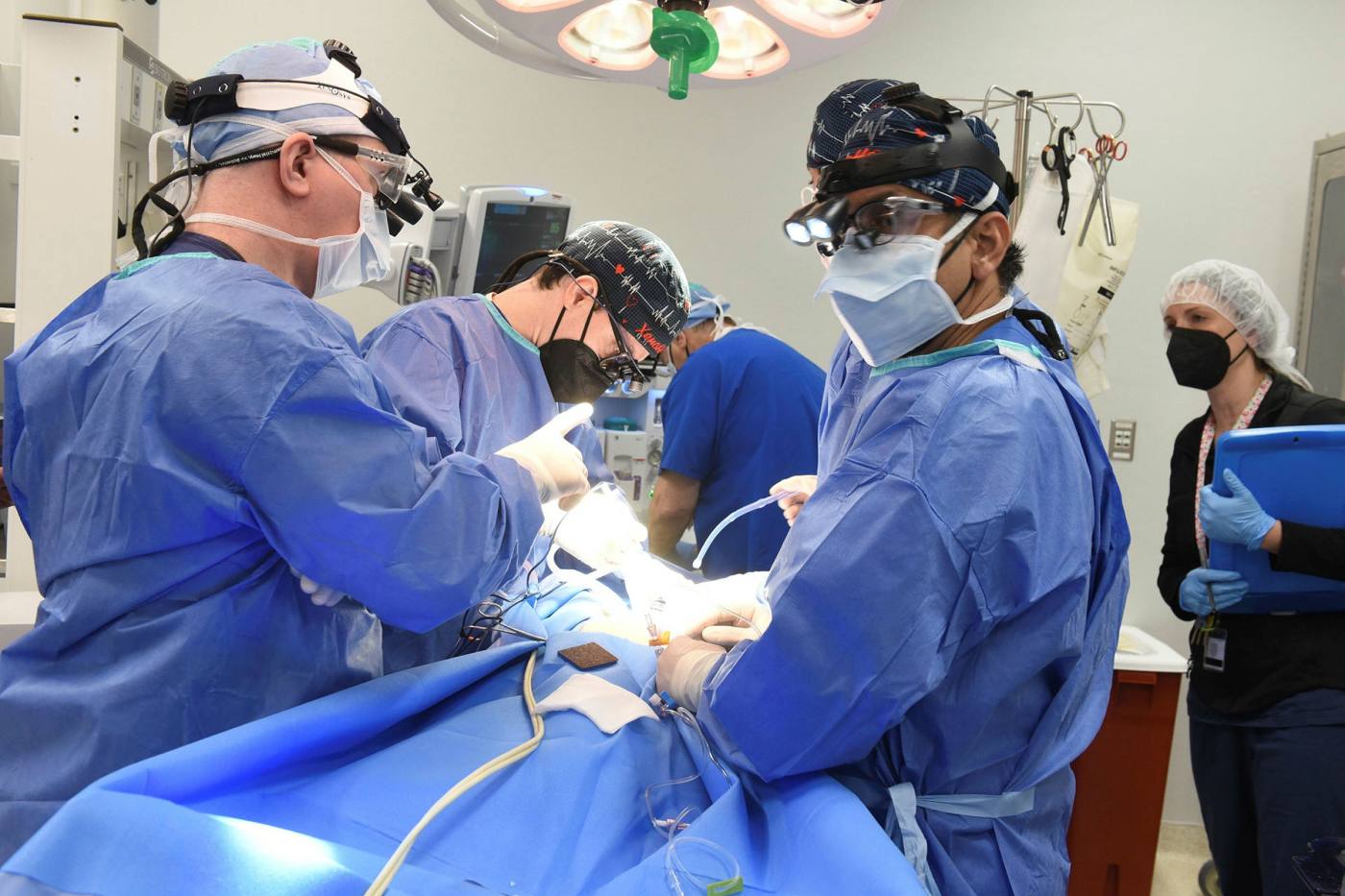

A revolution or a stop-gap?
革命還是權宜之計?
How long pig organs will work well in people remains to be seen. At present transplanted human organs typically suffer long-term damage as a result of incompatibility with the host’s immune system. But Mohiuddin is confident that such damage can be reduced by a new generation of drugs. He is treating patients including Bennett with an experimental compound called KPL-404 which Kiniksa Pharmaceuticals is developing as a treatment for autoimmune diseases.
豬的器官能在人體內正常工作多久還有待觀察。目前,被移植的人體器官通常會因與宿主免疫系統不相容而遭受長期損害。但穆希丁相信,新一代藥物可以減少這種損害。他正在用一種名爲KPL-404的實驗性化合物治療包括班尼特在內的病人,Kiniksa製藥公司正在開發這種化合物,用於治療自身免疫性疾病。
If xeno becomes a routine medical service, it will transform the economics of transplantation. The companies producing pig organs will make money by selling them to hospitals, while human organs are donated without payment. But other costs will fall because surgery can be scheduled in a predictable way rather than in an emergency when a donor organ becomes available.
如果異種成爲一種常規醫療服務,它將改變移植的經濟學。生產豬器官的公司將透過出售給醫院賺錢,而人體器官是無償捐獻的。但其他費用將會下降,因爲手術可以以可預測的方式進行,而不是在有供體器官的緊急情況下進行。
“There’s a value proposition here beyond just the supply of organs,” says Curtis of eGenesis. “You can increase the quality, robustness and the outcomes, revolutionising how transplantation is considered,” he says. “The cost savings will be enormous if we can remove the huge burden of renal dialysis from the healthcare system.”
eGenesis公司的柯蒂斯說:「除了提供器官,還有其他價值主張。」他說:「你可以提高質量、健壯性和結果,徹底改變移植的考慮方式。如果我們能從醫療系統中消除腎透析的巨大負擔,節省的成本將是巨大的。」
Meanwhile the ethical debate over breeding animals to harvest human body parts is much less intense now than it was when the first xeno experiments took place in the late 20th century. Feelings of disgust when new biological procedures are introduced — the “ugh factor” — usually fade with time, says Professor Michael Reiss of University College London, who serves on the Nuffield Council on Bioethics. That has happened with the first human-to-human transplants and with IVF babies, and seems to apply to xeno too, Reiss says.
與此同時,與20世紀後期第一次進行異種動物實驗時相比,現在關於繁殖動物以獲取人體器官的倫理爭論已經不那麼激烈了。在納菲爾德生物倫理學委員會任職的倫敦大學學院的邁克爾·賴斯(Michael Reiss)教授說,當引入新的生物療法時的厭惡感——「令人作嘔的因素」——通常會隨著時間的推移而消失。賴斯說,這在第一次人與人之間的移植和試管嬰兒中都發生過,而且似乎也適用於異種移植。
The mainstream view among bioethicists is that xenotransplantation is acceptable as long as the animals are well cared for. “Parts of animals, including heart valves, have been used for human surgery for decades,” Reiss points out.
生物倫理學家的主流觀點是,只要動物得到很好的照顧,異種移植是可以接受的。「動物的部分器官,包括心臟瓣膜,已經用於人類外科手術幾十年了,」賴斯指出。
Religions that forbid eating pork are generally willing to accept the use of pigs to save lives, Reiss adds. “Jewish authorities decided a long time ago that it’s acceptable to use pig parts for human transplants,” he says. “Islam doesn’t have quite the same structure of authority as Judaism and Christianity, but significant Islamic ethicists have said there’s nothing that forbids it, so I imagine it would come down to individual patients.”
禁止喫豬肉的宗教通常願意接受用豬來拯救生命,賴斯補充說。他說:「猶太當局很久以前就決定,用豬的器官進行人體移植是可以接受的。伊斯蘭教的權威結構與猶太教和基督教不太一樣,但重要的伊斯蘭倫理學家曾說過,沒有什麼東西禁止這樣做,所以我想,這應該由患者個人自己決定。」
“The consensus [among ethicists] is that there is nothing in principle to prohibit xenotransplantation from pigs,” agrees Mohiuddin, Maryland’s xenotransplant surgeon, who is Muslim.
"[倫理學家]的共識是,原則上沒有什麼可以禁止從豬身上進行異地移植。「馬里蘭的異地移植外科醫生穆希丁同意,他是穆斯林。
Mohiuddin faced another unexpected ethical objection to his pioneering operation when it turned out that the patient was sentenced to 10 years in prison for a stabbing in 1988. The family of David Bennett’s victim, who was paralysed and died prematurely, said Bennett was not a deserving recipient of the new heart. But Mohiuddin disagrees.
穆希丁的開創性手術還面臨著另一個意想不到的倫理問題,那就是這位病人在1988年因持刀傷人被判10年監禁。大衛·班奈特的受害者因癱瘓而過早死亡,他的家人表示,班奈特不配接受新心臟。但穆希丁不同意。
“We don’t check the patient’s background,” Mohiuddin says. “We choose our patients purely on clinical grounds, according to their need and suitability for the operation.”
「我們不檢查病人的背景,」穆希丁說。「我們純粹根據臨牀情況選擇患者,根據他們的需要和手術的適宜性。」
The breakthrough in xenotransplantation offers a glimmer of hope to the many patients around the world on waiting lists for new organs. But it’s far from the only technology being pioneered to make up for a shortfall in donations.
異種移植的突破給世界各地等待移植器官的患者帶來了一線希望。但這遠遠不是唯一一項用來彌補捐贈不足的技術。
Professor Robert Lechler of King’s College London, an immunologist whose research focuses on increasing tolerance of transplanted organs, says growing new organs from human stem cells, preferably from the recipient to avoid the risk of rejection, may be another long-term solution. An even more immediate prospect, he says, may be regenerative medicine within the body, restoring failing organs to good health so that no transplant is needed.
倫敦國王學院(King』s College London)的免疫學家羅伯特•勒克勒(Robert Lechler)教授的研究重點是提高移植器官的耐受性。他說,用人類幹細胞培育新器官,最好是從接受者身上提取,以避免排斥反應的風險,可能是另一個長期的解決方案。他說,更直接的前景可能是體內再生醫學,將衰竭的器官恢復到健康狀態,這樣就不需要移植了。
His colleague Mauro Giacca, professor of cardiovascular sciences at King’s, has discovered how to generate new muscle cells, myocytes, in the heart by applying short genetic control molecules called microRNA. Animal tests suggest that triggering a proliferation of myocytes after a heart attack could prevent subsequent heart failure and the need for a transplant. “The plan is to reach patients in two and a half or three years,” Giacca says.
他的同事、國王學院心血管科學教授毛羅·吉甲卡(Mauro Giacca)已經發現瞭如何在心臟中產生新的肌細胞,即心肌細胞,方法是利用一種名爲microRNA的短基因控制分子。動物實驗表明,在心臟病發作後觸發肌細胞增殖可以防止隨後的心臟衰竭和移植的需要。吉甲卡說:「我們的計劃是在兩年半或三年的時間內讓患者用上它。」
So for now, the jury is still out on whether xenotechnology is a game-changer — or simply a stop-gap solution. “It’s quite possible that we will not need pigs’ hearts or kidneys in 10 or 20 years’ time,” says Reiss. “Xeno may be seen then as a fascinating sideline that led nowhere because it was overtaken by other approaches. Or it may be making an important contribution to healthcare.”
因此,到目前爲止,異種技術究竟是一種改變遊戲規則的技術,還是一種權宜之計,還沒有定論。「很有可能在10年或20年的時間裏,我們將不再需要豬的心臟或腎臟,」賴斯說。屆時,異種移植可能會被視爲一種引人入勝的副業,但最終不會有什麼結果,因爲它被其他方法所取代。但它可能也會對醫療保健做出重要貢獻。」